The periodic table has been a permanent fixture of understanding chemical elements and their properties since it was first created by Russian professor Dmitri Mendeleev in 1869.
At the time, Mendeleev “saw in a dream a table where all elements fell into place as required. Awakening, immediately wrote it down on a piece of paper.”
Arranging the 63 elements then known to scientists, “according to the value of their atomic weights, presents a clear periodicity of properties,” leaving spaces on the chart for when more elements would be discovered. Today there are 118 elements included in the table.
Now, 150 years later, a team of scientists, led by Professor Uri Banin at the Hebrew University of Jerusalem’s Institute of Chemistry and Center for Nanoscience and Nanotechnology, are reinventing the concept of the periodic table, but for artificial atoms.
Banin explained to The Jerusalem Post that they are using this periodic table concept as an analogy.

“In analogy to what we know from atoms, we can now combine different quantum dots (‘artificial atoms’) to form molecular-like structures. This concept gives us a richness of possibilities that can emerge,” he said, adding that “it catches the imagination and enriches significantly the family of quantum dots based materials.”
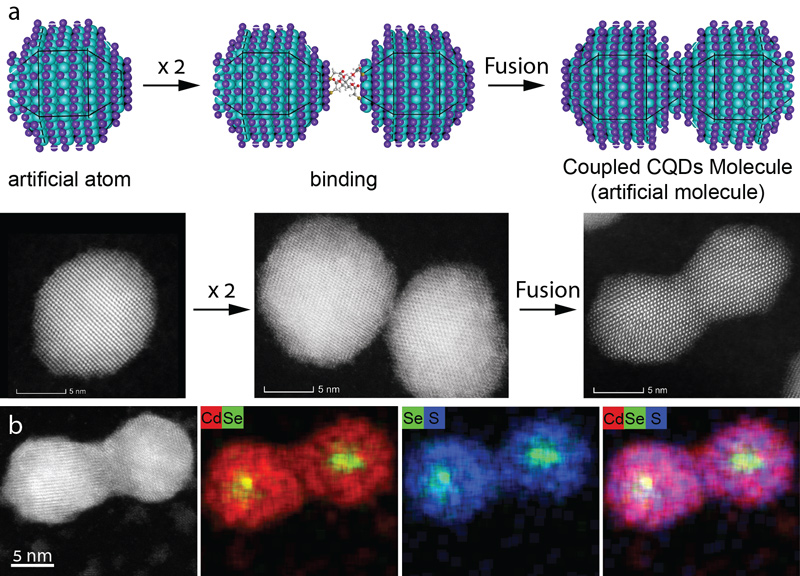
The nanoscience research team has developed a method that enables quantum dots to join together and form new molecular-like structures.
“Quantum dots are nano-sized chunks of crystal, each containing hundreds to thousands of semiconductor atoms,” Hebrew University explained in a statement. “When viewed through an electron microscope, they look like dots.
“As with real atoms, when you combine such artificial atoms together, they create a new (artificial) molecule with unique properties and characteristics,” the statement said.
These molecules are called “artificial” because they don’t form part of the 150 million original molecules that have been created through combining atoms from the 118 known elements in Mendeleev’s Periodic Table, which today is an integral part of the scientific world.
Interestingly, quantum dot atoms are mercurial, meaning that their physical, electronic and optical properties change when their size changes.
Banin highlighted that “a larger quantum dot will emit a red light, while a smaller one, of the same material, will emit a green light.”
Together with his team, Banin has also formulated a method that gives scientists a chance to create new quantum dot molecules while still retaining control over their size and composition.
“I began considering the infinite possibilities that could arise from creating artificial molecules from artificial atom building blocks,” Banin said.
Over the last few decades, scientists have improved their understanding of the physical properties that make up quantum dots and have also learned how to manipulate their levels of control.
Banin told the Post that today, quantum dots are being used in our day-to-day lives in the biological and technological spheres.
“Quantum dots today are already being used for bio-imaging and bio-tracking,” he said, explaining that these ultra-small tags are used to help study biological objects by “following and tracking biomolecules and cells that are tagged with quantum dots.”
He added that the dots are also used “in sensing applications to measure or identify the presence of other molecules” and in solar energy.
In the technological sphere, Banin said that quantum dots are also “making an impact in flat panel displays including TV screens.”
“It enriches the color quality and at the same time also saves energy… and we may see much richer colors, now that we will have this ability to control quantum dots,” Banin said, adding that this will impact the thinking of the future.
“Considering the rich selection of size and composition among colloidal quantum dots, we can only imagine the exciting possibilities for creating a selection of artificial molecules with great promise for their utilization in numerous opto-electronic, sensing and quantum technologies applications,” he concluded.
The findings were published in the latest edition of Nature Communications.